Importance of pH in Daily Life

Importance of pH in Daily Life
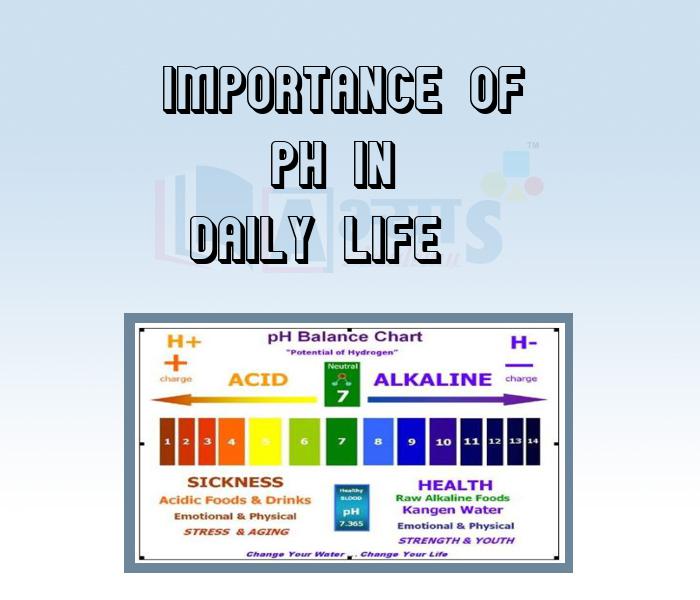
The pH values of some common solutions
How is pH measured?
The pH of a solution is generally determined with the help of a pH paper, or universal indicator. The pH paper gives a particular colour with a solution of particular pH. The colour is compared with a chart which has different colours at different pH values.

Role of pH in everyday life:
1. In our digestive system: Hydrochloric acid produced in our stomach helps the digestion of food without causing any harm to the stomach. But when the amount of the acid goes beyond a certain limit due to indigestion, pain and irritation are created in the stomach. So, in order to neutralize the effect of excess acid, a mild base called antacid is usually taken. Magnesium hydroxide (milk of magnesia) is a mild base which is usually used as an antacid.
2. Acids cause tooth decay: When we eat sugary food, it gets degraded by bacteria present in the mouth and an acid is formed. When the pH becomes lower than 5.5, tooth enamel gets corroded. Saliva, which is slightly alkaline, produced in the mouth neutralizes some acid, but excess acid remains unaffected. The excess acid can be removed only by the use of toothpaste which is alkaline. Neem stick contains alkaline juice. So, the cleaning of tooth by Neem stick also helps to reduce tooth decay.
3. Acid is produced in fatigued muscle: As a result of physical exercise, stiffness and pain in the muscle starts due to the formation of lactic acid. The supply of oxygen in the muscle is reduced. This causes difficulty in the release of energy leading to increase in the rate of anaerobic metabolism. As a result, lactic acid gets accumulated in the muscles.
4. Some animals and plants contain acids: Honey-bee injects an acid through its stings which causes pain and irritation. Hence, a mild base like baking soda is applied to treat the wound. Similarly, nettle leaves, which have stinging hairs, when touched inject formic acid in our body. This causes a burning pain. Wasp sting leaves an alkali. Hence, a wasp sting should be washed with a mild acid such as vinegar. Note Nettle is a stinging plant. When one accidentally touches its hairs, a painful effect is produced. As a remedy, the affected area is rubbed with the dock plant. The dock plant is alkaline which neutralizes the effect of the acid.
5. The brilliance of a tarnished copper vessel can be restored by using acid: You know, lemon juice contains an acid. In order to clean a copper vessel, we rub it with the piece of a lemon. The tarnish on the vessel is caused by the formation of a layer of basic copper oxide. Since lemon juice contains citric acid, it reacts with the copper oxide to form copper citrate and is washed away. The vessel then regains its shining appearance.
6. pH of soil: Soils are generally acidic. Plants require definite pH range for their proper growth. They do not grow in alkaline soil. Many plants do not grow properly in highly acidic or highly alkaline soil. So, highly acidic soil is treated by spreading quicklime, slaked lime or calcium carbonate to lower its acidity.
pH of Salts:
The aqueous solutions of all kinds of salts do not have the same pH value.
1. Salts of strong acids and strong bases: Sodium chloride (NaCl), potassium nitrate (), sodium sulphate (
), etc., are salts of this category. The aqueous solutions of these salts are neutral with pH value of 7.
2. Salts of strong acids and weak bases: Aluminium chloride (), copper sulphate (
), zinc sulphate (
), etc., are salts of this category. The aqueous solutions of these salts are acidic with pH value less than 7.
3. Salts of weak acids and strong bases: Sodium acetate , sodium carbonate
and sodium hydrogencarbonate
are examples of this category of salts. The aqueous solutions of these salts are basic in nature with pH value more than 7.
Solutions A,B C and D have pH 2,3,7 and 12 . Which of these has the highest acidic strength? | |||
| Right Option : A | |||
| View Explanation | |||
Which of the following are correct : (a) Saliva, which is slightly alkaline, produced in the mouth neutralizes some acid, but excess acid remains unaffected. (b) When the pH in mouth becomes lower than 5.5, tooth enamel gets corroded. (c) As a result of physical exercise, stiffness and pain in the muscle starts due to the formation of lactic acid. | |||
| Right Option : D | |||
| View Explanation | |||
Tooth decay starts when the pH of the mouth is lower than ________________ | |||
| Right Option : D | |||
| View Explanation | |||
Students / Parents Reviews [10]
My experience with Abhyas is very good. I have learnt many things here like vedic maths and reasoning also. Teachers here first take our doubts and then there are assignments to verify our weak points.

Shivam Rana
7thMy experience with Abhyas academy is very good. I did not think that my every subject coming here will be so strong. The main thing is that the online tests had made me learn here more things.

Hiya Gupta
8thIt was a good experience with Abhyas Academy. I even faced problems in starting but slowly and steadily overcomed. Especially reasoning classes helped me a lot.

Cheshta
10thAbout Abhyas metholodology the teachers are very nice and hardworking toward students.The Centre Head Mrs Anu Sethi is also a brilliant teacher.Abhyas has taught me how to overcome problems and has always taken my doubts and suppoeted me.

Shreya Shrivastava
8thA marvelous experience with Abhyas. I am glad to share that my ward has achieved more than enough at the Ambala ABHYAS centre. Years have passed on and more and more he has gained. May the centre flourish and develop day by day by the grace of God.

Archit Segal
7thAbhyas Methodology is very good. It is based on according to student and each child manages accordingly to its properly. Methodology has improved the abilities of students to shine them in future.

Manish Kumar
10thIt has a great methodology. Students here can get analysis to their test quickly.We can learn easily through PPTs and the testing methods are good. We know that where we have to practice

Barkha Arora
10thIt was good as the experience because as we had come here we had been improved in a such envirnment created here.Extra is taught which is beneficial for future.

Eshan Arora
8thAbhyas is a complete education Institute. Here extreme care is taken by teacher with the help of regular exam. Extra classes also conducted by the institute, if the student is weak.

Om Umang
10thOne of the best institutes to develope a child interest in studies.Provides SST and English knowledge also unlike other institutes. Teachers are co operative and friendly online tests andPPT develope practical knowledge also.
